Semiconductors: Current Trends You Can’t Live Without
My last blog showed how nearly everyone uses optics on a fairly regular basis (fiber optics = Internet, check it out: Why You Can’t Live Without Fiber Optics). The same is true for semiconductors, as came up in my Solid State Physics class this semester, taught by Dr. George Rybicki (one of the new faculty in the Physics & Space Sciences Department, with research in nuclear physics, radiation detection and measurement, and nuclear non-proliferation). Don’t be scared of the name; “solid state physics” means the study of solids through various methods, such as crystallography, electromagnetism, quantum mechanics, and so forth . . . ok, you can be a little scared.
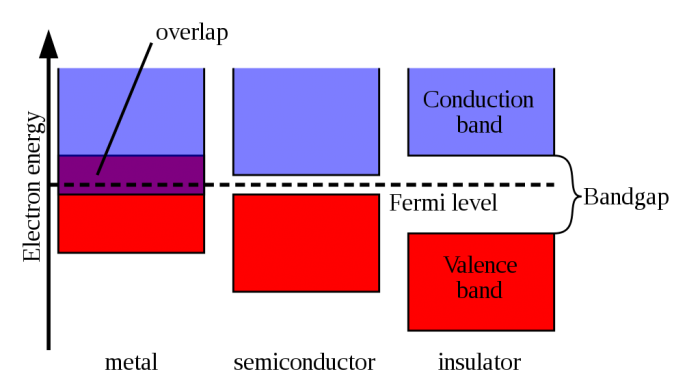
One part about solid state physics is semiconductors, which is just what it sounds like–“semi” and “conductor”, meaning it partially conducts a current. This is best illustrated by the diagram below. Current is caused by a flow of electrons, which are found orbiting the nucleus of atoms. The outer-most orbit is called the valence band, and these are the electrons that can get shared or transferred when interacting with other atoms (this is also how molecules form, which is what everything is made of—science!). When a material has energy added to it, the electrons begin to move. Notice below how for metals, the valence band and conduction overlap, meaning electrons move freely back and forth–hence metals conduct electricity very easily. An insulator is the exact opposite. The bands are very far apart (in microscopic scales, that is), and an inordinate amount of energy would have to be added to get the electrons enough energy to jump the gap (and often the material probably wouldn’t hold up under that kind of energy). So stuff like wood and rubber don’t conduct electricity. A semiconductor is right between the two; it has an energy gap, so the electrons don’t move as freely as in metals, but the gap is small enough that current will begin to flow after a certain amount of energy has been reached.
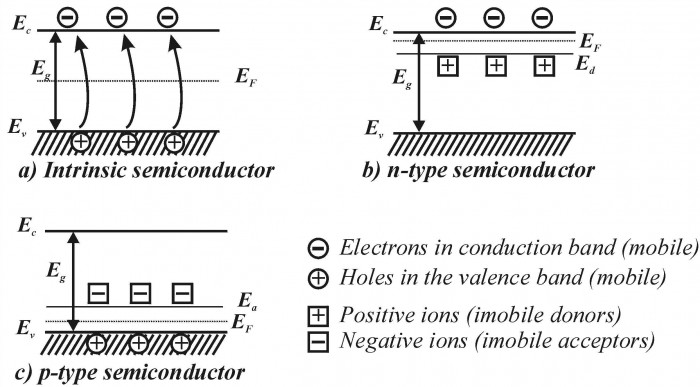
A semiconductor’s natural energy gap gives what is called an internal electron concentration, which specifies how much current the material will conduct (also called a pure semiconductor). However, the great thing about semiconductors, and why they’re so useful, is we can add others substances (called impurities) to the material (called “doping”; I know, I laughed when I first heard the term, too). This will give an external electron concentration, a concentration we choose based on the dopant, and hence we can control how much conductivity we need.
For example, when antimony is added to silicon, an extra electron is added since silicon has four valence electrons to share with antimony, while antimony has five valence electrons. This creates a donor-type semiconductor, aka “n-type”. Likewise, if the dopant has only three valence electrons, as is the case for boron, then a “hole” is created, which is exactly the same as the donor electron except for positive, creating an acceptor-type semiconductor, ask “p-type”. This extra electron is now stored in the “donor level”, while any holes are stored in the “acceptor level”. Whichever one it is, the electron or hole concentration has been increased, meaning an increase in conductivity.

Why do we care? Well, as uk.rs-online.com says in one of their website articles, the electronic devices that rely on semiconductors ranges from extremely sophisticated, expensive medical equipment to the cheapest transistor radio, televisions, computers, and gaming consoles being included in this range. Basically, “it would be more difficult to find an electrical component or product that doesn’t contain a semiconductor among its workings than it would be to find one that does.” I don’t know about you, but I don’t really want to give up any of my electronics, cheap or otherwise, so I, at least, cannot live without semiconductors, and I’m willing to bet quite a few people would agree.
References:
https://en.wikipedia.org/wiki/Semiconductor#Doping
https://en.wikipedia.org/wiki/Doping_(semiconductor)