How Does Your Car Use Thermodynamics?
I always find it fun to learn about applications of the things I’m learning in lecture. My thermodynamics class, taught by Professor Durrance, has been doing a really good job of that this semester.
We recently learned about two cyclic processes called the Otto Cycle and the Refrigeration Cycle that describe the physics of how some of our everyday objects run; a “behind the scenes” look into our cars and refrigerators, if you will. This is a very simplified (not to mention, idealized) run-through, but I still found it interesting.
The Otto Cycle
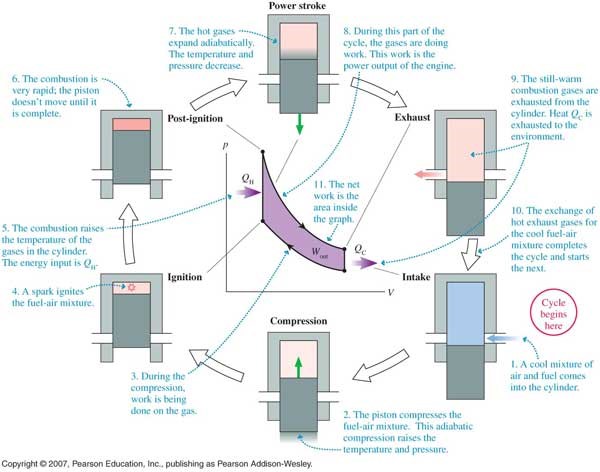
The Otto Cycle can be used to describe how an internal combustion engine works. It explains how your car can run by just filling it up with gas. The first thing to know is this cycle is based on what we call the ideal gas law. It is written as PV=NkT, where P is pressure, V is volume, T is the temperature, N is the number of molecules. K is the Boltzmann constant (an observed quantity). We’ll only be worrying about the pressure, volume and temperature; notice if pressure increases, then to keep the equality true, either volume must decrease or temperature must increase (or a combination of both). Similar relations are true for changing volume and for changing temperature.
These relations are important for understanding an internal combustion engine because the engine’s main component is a piston. The piston a metal piece that moves up and down inside a cylinder, compressing and expanding the gas.
The second thing to know about thermodynamics
The second thing to know about thermodynamics is the relation we refer to as “adiabatic.” An adiabatic process means no heat or matter is transferred in or out of the system, accomplished either by a really fast reaction or the system has been sufficiently isolated from everything else.
Putting the ideal gas law, the piston, and adiabatic processes together with some fuel, we get an engine! More details are listed in the pressure vs. volume diagram below, but basically, the fuel enters the cylinder and is adiabatically compressed. This means the volume decreases, the pressure and temperature increase. When compression is complete, the spark plugs ignite the fuel, which then increases the temperature and pressure at a constant volume. Then the gas expands adiabatically, called the “power stroke,” resulting in a sudden drop in the pressure and temperature. This part of the reaction is what gives power to the car. And finally exhaust fumes are released, decreasing the pressure a bit more, and the cycle starts over with a new batch of fuel.
(Photo Credit)
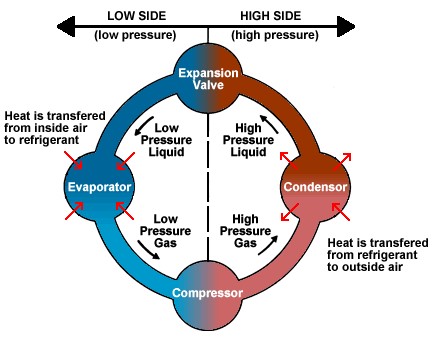
The Refrigeration Cycle…
also depends on compression/expansion and phase changes between liquid and gas. The process starts by gas being adiabatically compressed, increasing the pressure (but with no change in heat). All the gas is then sent through a condenser, turning all into liquid and excess heat being expelled out of the refrigerator. That is why you feel warm air coming out of your fridge!. The liquid then goes through a specially designed valve, called either a “throttle valve” or an “expansion valve.” The valve decreases the pressure while volume increases (aka expansion). The final step is for the liquid to be evaporated into a gas once more by taking heat from the air inside the refrigerator. This results in the air being cooler inside. Then the cycle repeats.
(Photo Credit)
So there you have it. A few basics of thermodynamics that have influenced your entire life and you probably didn’t even know it! I honestly didn’t think I was going to like this class, but it’s turning out to be quite fascinating, especially when I can see the application. It is always reassuring when things I am learning at Florida Tech are actually relevant to the real world.
Featured photo credit: TCServiceCenter.com
%CODE1ASTRONOMYASTROPHYSICS%